Technologies
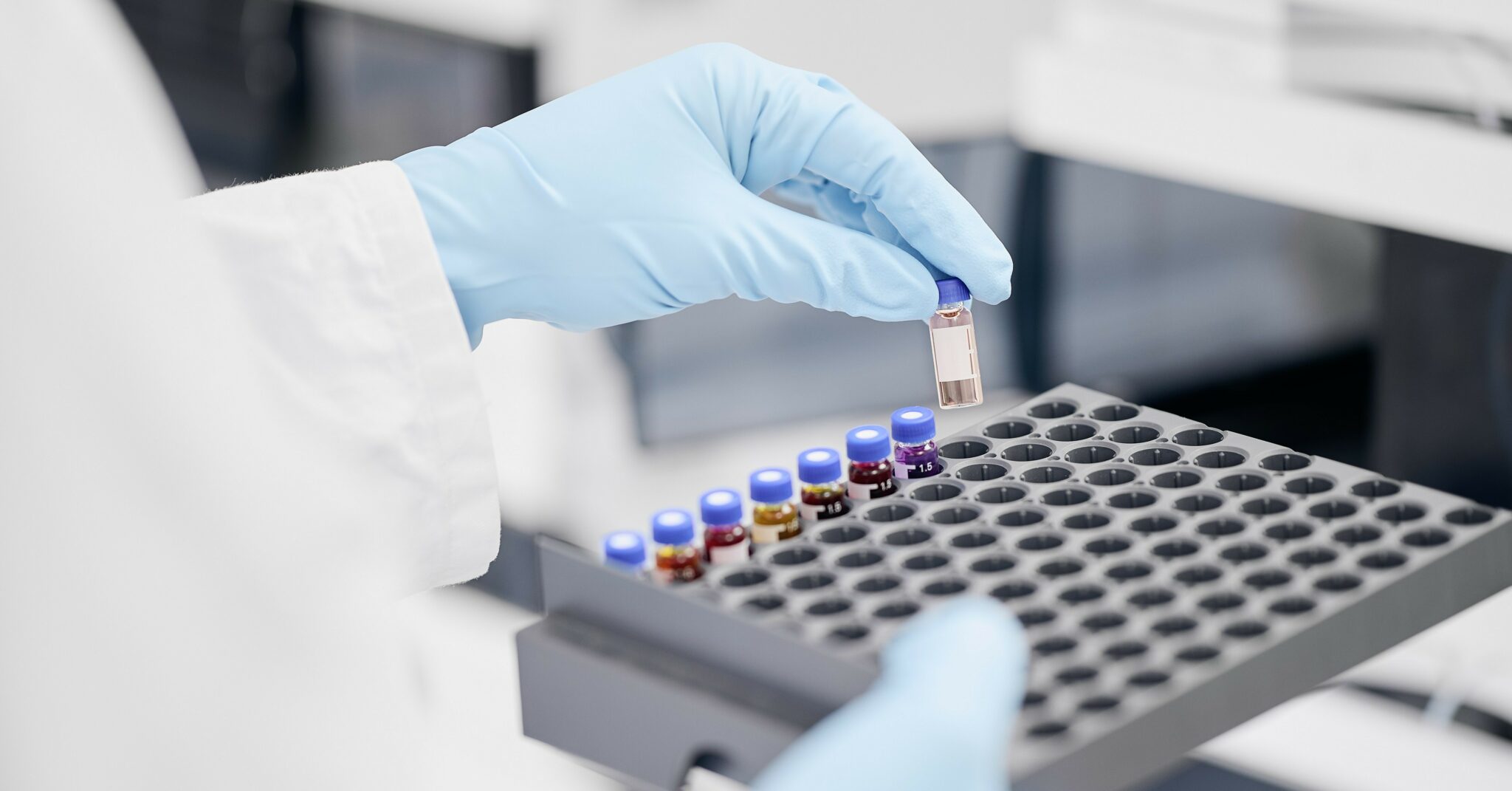
Our state-of-the-art laboratories are well equipped, and offer a wide range of analytical techniques, such as:
- HPLC (DAD, RI, MWD Detector; FLD)
- UPLC
- GC
- Particle sizer for wet measurement
- Particle size distribution
- Optical rotation
- Viscosity measurement
- Density measurement
- Microscopic determinations
- TLC
- NIR spectroscopy
- FTIR spectroscopy
- UV/Vis spectroscopy
- Dissolution testing
- Water content
- pH
- Osmolarity
- Laser absorption (head space analysis)
- Sieve analysis
- Microbial testing